TB vaccine and test move to next phase in England and Wales
 ©Tim Scrivener
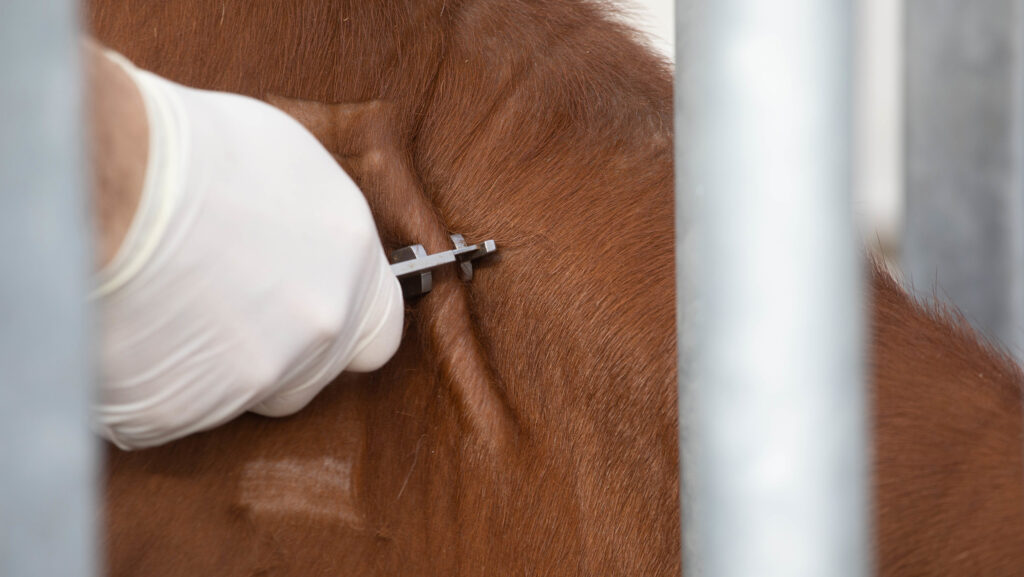
©Tim Scrivener Commercial cattle farms in low-incidence bovine tuberculosis (TB) areas of England and Wales are being invited to participate in the next round of field trials for a cattle vaccine and new skin test.
The Animal and Plant Health Agency (Apha) is looking for interested farmers and vets, who are being encouraged to volunteer and support the delivery of the project as it moves into Phase 3 trials.
See also: Experts to review new evidence in battle against bovine TB
To be eligible, herds must be officially TB Free (OTF), from the Low-Risk Area (LRA) of England or the Low TB Area (LTBA) of Wales, and have been in existence for eight years or more.
They must not currently be in an enhanced surveillance testing regime, such as radial or contiguous TB testing, and not be in an active TB hotspot (as defined by Apha).
Cattle herds must also have been OTF for a minimum of three continuous years with a herd tuberculin skin test completed within three calendar years, and not have introduced cattle from a higher TB risk area in the 12 months prior to the enrolment date.
Bovine TB costs taxpayers in England about £100m every year, with an estimated further £50m cost to the industry. More than 60,000 cattle in England and Wales were slaughtered during 2023-24 to tackle the disease.
© Tim Scrivener
Confidence
Apha is confident that if this next phase is successful, the industry will be a step closer to a vaccine which can be used in conjunction with other measures to tackle the disease.
UK chief veterinary officer Christine Middlemiss said: “These trials and the active participation of farmers will help us in ensuring any new vaccine and testing approach is both effective and practical.”
Laboratory studies have indicated that both the cattle BCG vaccine and Diva (detecting infected among vaccinated animals) skin test are safe and the test performs well under controlled Apha facility environments.
Recent international studies investigating the full extent of BCG protection in natural conditions found a total efficacy of 89%.
Apha chief executive Jenny Stewart said this round of field trials was a significant step forward in developing a viable and effective cattle TB vaccine, and urged those who meet the criteria to contact Apha if they are interested in participating.
Further information on the field trials can be found on the TB Hub.
