Europe’s first gene-edited wheat trials see breakthrough
 © Crops Science Centre
© Crops Science Centre Preliminary results from Europe’s first field trials of gene-edited (GE) wheat have indicated there’s no yield or other agronomic penalty from the precision-breeding technique.
This means that precise edits can now be made to a wheat genome to improve its performance and specific grain qualities that currently take many years to change using conventional breeding techniques.
See also: Why breeders are looking at the past for tomorrow’s wheats
“This has been the first real test of the technology in wheat in the field in Europe, and it’s a very significant finding,” notes Nigel Halford, whose team at Rothamsted Research carried out the landmark trials.
“There are many agronomic factors we’re still analysing, but there’s no great difference in yield, although thousand grain weight (TGW) appears slightly reduced. What’s also significant is that grain sulphur levels have actually improved.”

Nigel Halford © Tom Allen-Stevens
Field trials
The field trials sown last October were set up to test a wheat that has been altered using Crispr-Cas9 editing to have very low levels of asparagine.
“This is an amino acid that occurs naturally in cereals, but during cooking at high temperature, such as baking or toasting, asparagine is converted to acrylamide,” explains Prof Halford.
“This is a carcinogen and the EU introduced benchmark levels on how much acrylamide is allowed in wheat-based food, such as breakfast cereals, bread and biscuits, in 2017.
“These rolled over into UK law after Brexit. The EU is already considering going further by imposing maximum levels, over which it would be illegal to sell a product.”
The variety on trial was Cadenza, with lines that had the gene responsible for most asparagine accumulation in the grain “knocked out” using the precision breeding technique.
Also in the trial was Claire, where the gene had been knocked out by treating seeds with a chemical – a much older technique, targeting induced local lesions in genomes, sometimes known as TILLING.
Although this is viewed as a conventional breeding technique, it’s a more laborious process and much less precise, explains Sarah Raffan, who works alongside Prof Halford at Rothamsted.
“The TILLING lines yielded significantly lower than the control lines of Claire we had in the trial,” she says (see chart).
“They also had a lower TGW, while NDVI [normalised difference vegetation index] scans through the season suggested lower biomass.
“TILLING introduces tens of thousands of random mutations in each seed, almost all unrelated to the target gene.
“It takes many crosses to get rid of these, and any that are close to the target gene may be difficult or impossible to cross out. It’s likely that this genetic baggage caused the performance lag.”

Sarah Raffan © Tom Allen-Stevens
Higher sulphur
All of the mutated lines, whether GE or TILLING, showed higher levels of grain sulphur, which helps to lower levels of acrylamide.
Full analysis of the samples has yet to be completed, including confirmation of asparagine levels, but the trials are already seen as a “fantastic step forward” for GE in the UK.
“By using this intervention, we’ve achieved a triple knock-out,” explains Dr Raffan.
“This is where sections of the ASN2 gene have been removed on all three of the wheat genomes, without any other change to the host plant.
“It has an asparagine level of just 30% compared with a non-edited comparison. We also have lines with an additional edit in an ASN1 gene, and these have even lower asparagine levels.
“These will be included in this year’s field trial.”
The selected TILLING lines, however, each had a mutation in just one of the ASN2 genes, Dr Raffan continues.
“One of our breeder partners has been stacking the mutated genes in the Claire background for us.
“This is extremely laborious and time-consuming, and we only had partial knockouts in the first field trial. We will be including triple TILLING knock-outs in year two, although the yield penalty is a concern.”
Provided all goes well with the tests on the material from this year’s harvest and with subsequent trials, GE germplasm could be handed to breeders.
Several major UK breeders are partners in the project, so theoretically the trait could be introduced across UK bread and biscuit wheats.
“However, this will only happen if appropriate regulations are put in place, that are science and risk-based,” notes Prof Halford. “Otherwise plant breeders cannot be expected to make the investment”.
Low asparagine varieties could start appearing in National List trials in as little as five to 10 years’ time, says Dr Raffan.
“Had the regulations changed sooner, we could have done field trials earlier, but we are glad to see that things are now moving.
“The advantage of GE is that you can introduce changes in just one generation, while making the same improvement using conventional techniques may take many years or in some cases may not be possible at all.
“At the moment, we have only made the edit to a limited number of varieties, but it is now possible to edit most wheat varieties, or once an edit has been made the trait can be crossed into any breeding programme using conventional breeding.”
The team is also researching other amino acids, she continues. “We’ve just started looking at raising levels of lysine, using our low asparagine wheat as the starting material.
“Lysine is an important constituent of animal feed, especially for pigs and chickens, and is mainly provided through imported soya.
“If all goes well, using GE we could have high-lysine wheat in field trials within the next five years.”
How the gene-edited wheat performed
The three GE lines of Cadenza (marked as GM in the chart) yielded the same as the unedited control, but the TILLING lines yielded significantly below the control, Claire.
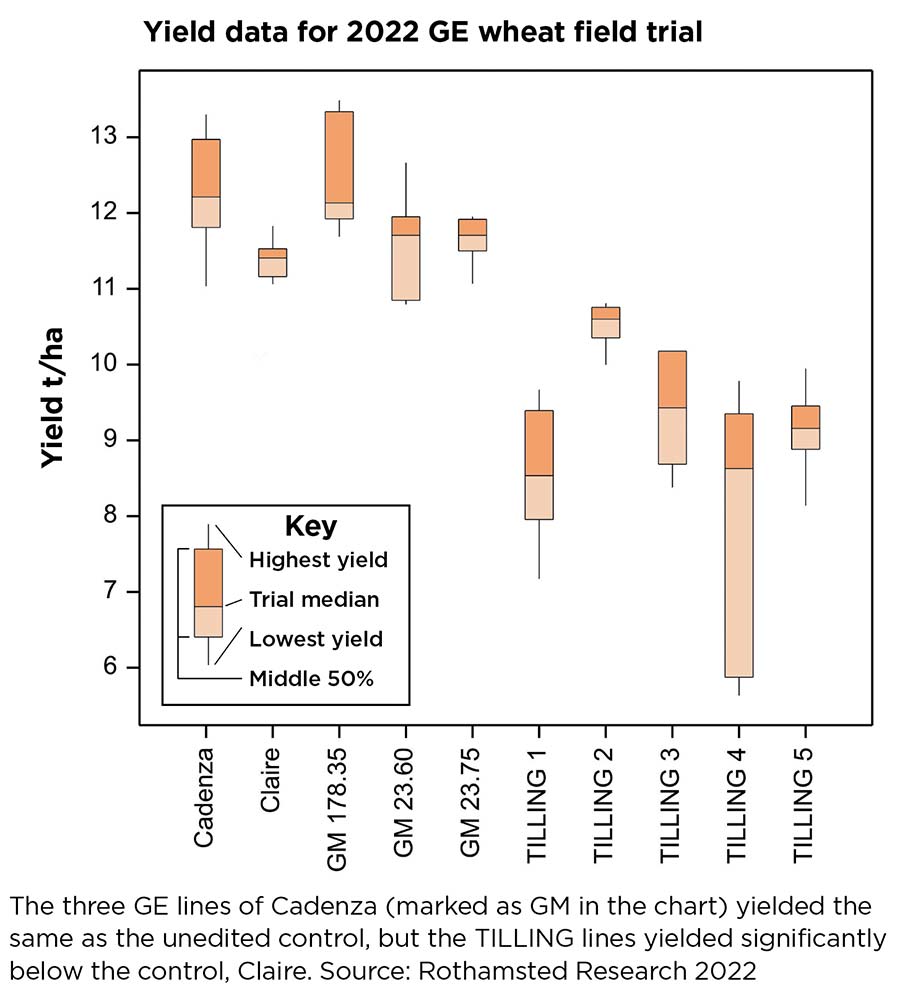
Source: Rothamsted Research, 2022
Achieving the ‘triple knock-out’
The gene responsible for most of the production of amino acid in the grain, asparagine synthetase 2 (ASN2), was discovered several years ago.
“There are actually five asparagine synthetase genes, but ASN2 is by far the most active in the grain,” notes Prof Halford.
It’s possible, through marker-assisted breeding, to select lines without the gene, but there’s a snag with attempting to do this conventionally.
“Wheat is hexaploid, which means it has three copies of its genome, that we refer to as A, B and D,” he continues.
“Many varieties, including Claire, lack an ASN2 gene on the B genome due to a natural mutation that occurred before wheat was domesticated. Ironically, this was a much bigger genetic event than anything we have done by GE.
“Breeders could select lines carrying this natural mutation, but to produce a variety lacking the gene on all three genomes through conventional techniques would be impossible.”
And this is why Prof Halford and his team have turned to GE to alter the plant DNA. Crispr-Cas9 is a GE technique whereby GM is used to introduce genes encoding short RNA sequences (guide RNAs, or gRNAs) into the host plant, alongside a gene encoding Cas9 enzyme.
The gRNAs recognise specific stretches of genetic code, targeting the Cas9 enzyme to the target gene, where it cuts the DNA.
The cell tries to repair the damage, and that’s when the mutation occurs.
By using different gRNAs and techniques, researchers can deactivate or alter – edit – specific parts of the genome, thereby conferring traits, such as low asparagine. Once the edit is done, the GM components can be crossed away using conventional breeding techniques.
The Rothamsted team successfully edited the Cadenza DNA to deactivate the ASN2 gene on all three copies of its genome – a ‘triple knock-out’.
But one of the hold-ups in the development of the GE lines has been regulation.
“Field trials are a crucial step in the development of any new trait – although we’ve grown the GE lines in the lab, it’s not until you put them in the field that you know how they will perform yield-wise or interact with their environment,” explains Prof Halford.
“We also need to know whether the edit causes changes to grain proteins.
“But GE crops have been treated as genetically modified organisms under EU regulations, which makes trials difficult and prohibitively expensive.
“These regulations rolled over into UK law at Brexit, and our first field trial was run under those regulations.”
Law change
However, the UK government has passed a statutory instrument that changed the law to make field trials of GE plants much easier, as long as any GM elements are not present in the plants.
“We now have some plants from our edited lines that are GM-free and are, therefore, ‘qualifying higher plants’ under the new field trial rules.
“We can grow those plants anywhere as long as we notify Defra, and we will be bulking up the seed for a full field trial in 2023-24,” says Prof Halford.
The new rules on field trials came into force in March this year, and events are now moving apace.
The Genetic Technology (Precision Breeding) Bill that is passing through parliament will set out new regulations aimed at facilitating the commercial breeding and marketing of GE plants, or precision-bred organisms, as they are referred to in the bill.
“This is a massive, long-overdue step forward for crop biotechnology in the UK,” he says.
“The technology is developing very fast: there are new Crispr techniques that don’t involve a GM step at all.
“What’s more, there are methods that use a DNA template to change a gene so that it still makes a functional protein, but some characteristics of the protein are changed, rather than just knocking the gene out.
“So it is a very exciting time to be involved in plant biotech,” he concludes.
Trials shed light on mycorrhizal interaction

Tom Thirkell © Tom Allen Stevens
Initial results from the first gene-edited barley trials in the UK have demonstrated the strong interaction between cultivated crops and arbuscular mycorrhizal fungi (AMF).
Scientists at the Crop Science Centre in Cambridge have identified a nodulation signalling pathway gene (NSP2) that allows plants and microbes to interact.
This was knocked out on gene-edited (GE) lines of Golden Promise barley, and grown in plots in the field alongside GM lines where the gene had been overexpressed.
“Preliminary analysis suggests the trials performed as we expected, confirming results we’d seen in the lab,” reports Tom Thirkell, research associate at the Crop Science Centre.
“We’ve yet to assess all the data, but initial nutrient analysis from the trials appears to have confirmed the role played by this important gene.
“We’ve yet to assess all the data, but the trials appear to have confirmed the role played by this important gene.”
It’s the first time these genotypes have been grown in the field, explains Dr Thirkell.
“Every growth stage we reached was a celebration in itself. To bring the plots to harvest, especially in the challenging dry conditions, was a great success.”
The team at Crop Science Centre, led by Giles Oldroyd, have been exploring the signalling pathways plants have with naturally occurring AMF.
It’s thought the symbiotic relationship, which goes back over 400m years, is similar to the one that allows legumes to interact with nitrogen-fixing bacteria.
Unlocking the genetic secrets to these signalling pathways could pave the way to cereals that can fix their own nitrogen and achieve high yields without synthetic inputs.
“This is very much the first stage of our work, but these results are super-exciting,” notes Dr Thirkell.
“We’ll be repeating the trials, this time at two locations, and we look forward to learning so much more about AMF.
“Ultimately, we’ll also be gaining a greater insight into the massively complicated genetic mechanisms that allow plants and microbes to interact.”

